7. Description
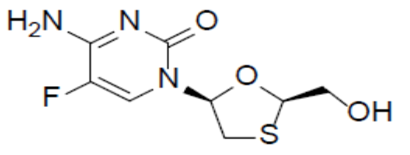
Emtricitabine (FTC)
Emtricitabine (FTC), a synthetic nucleoside analog of cytidine, is an HIV nucleoside analog reverse transcriptase inhibitor (HIV NRTI). It is chemically described as 4-amino-5-fluoro-1-(2Rhydroxymethyl-1,3-oxathiolan-5S-yl)-(1H)-pyrimidin-2-one with a molecular formula of C8H10FN3O3S and a molecular weight of 247.24 g/mol. The structural formula as:
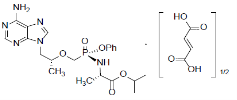
Tenofovir Alafenamide (TAF)
TAF, an HIV NRTI, is converted in vivo to tenofovir, an acyclic nucleoside phosphonate (nucleotide) analog of adenosine 5′-monophosphate. It is chemically described as L-alanine, N-[(S)-[[(1R)-2-(6-amino-9H-purin-9-yl)-1methylethoxy]methyl]phenoxyphosphinyl]-, 1-methylethyl ester, (2E)-2butenedioate (2:1) with a molecular formula of C21H29O5N6P•½(C4H4O4) and a molecular weight of 534.50 g/mol. The structural formula as: